Classification Of Elements
Classification of Elements:Classification of elements related to arrangement of elements in a specified order .This order can be according to the atomic mass of an atom or atomic number of atoms. Firstly let us see what is the element ? Element is the composition of single type of atoms . For example Copper metal is consist of only copper atoms, Silver metal is consist of silver atoms. So composition of single type of atoms is called element.In the modern time we have 116 elements in our nature in addition to man-made.
Atomic Number:Atomic number is defined as the number of protons or number of electrons in an atom.For example atomic number of zinc atom is 30 , it means zinc atom contains 30 protons and also 30 electrons.
Atomic Mass: Atomic Mass of an atom is defined as the summation of total number of protons and neutrons present in an atom . For example Atomic Mass of Oxygen is 16 , it means oxygen has 8 protons and 8 electrons in its atom.
The purpose of defining atomic mass and atomic number here is to that in the next terms when i will use terms atomic number and atomic mass you will got it know already that what these are in actual.Lets come back to classification.
Classification determines the arrangement of atoms in specific order in a table.The elements are arranged either according to their mass or atomic number so that elements of similar properties get together in a group.The table of ordered elements is called periodic table. It is called periodic table because it contains the elements which have similar properties after a regular interval of elements.The purpose of periodic table is to get elements of similar properties in a group.
Groups: Elements arranged in a vertical columns are called groups.
Periods: Elements arranged in horizontal rows are called periods.
Several scientists had come to give their periodic tables but these were rejected due to some reasons . Lets have a small view on previous definitions of periodic table of different scientists.
Doberenier Traid:Doberenier described that each mass average of first and third element in the group of periodic table is equals to mass of 2nd element.For example Li Na K.These are the symbols of Lithium, Sodium and Potassium elements respectively. Mass of Lithium is 7 and of potassium is 39 .Taking average (7+39)/2= 23 which is the mass of sodium atom.This rule was failed because it was not valid for all those atoms in which one is of low mass and other is of high mass secondly it was based on only atomic mass and not all elements can't be arranged in triads.
Newland's Octave Rule: Newland described that each eight element in periodic table has the same properties as of first element. This rule got very famous but it was not satisfactory for all of elements.Newland Octave rule was valid for all those elements having atomic mass less than 20. So it was not valid for those having mass more than 20 .Therefore it was failed.
Mendleve's periodic table:Mendleve had described periodic table in which elements were arranged in order of increasing atomic mass. This table got high popularity as many of elements arranged in atomic number were in a group of similar properties which was the base rule of periodic table.But it had a fault in it that if we consider elements according to their properties than the order of mass was not followed. For example properties of potassium is similar to sodium so it was placed below sodium atom but mass of potassium is 39 than argon is 40 still argon is placed before potassium due to consideration of properties . Therefore here order of mass could n't be followed.See here is the image of mendleve's periodic table.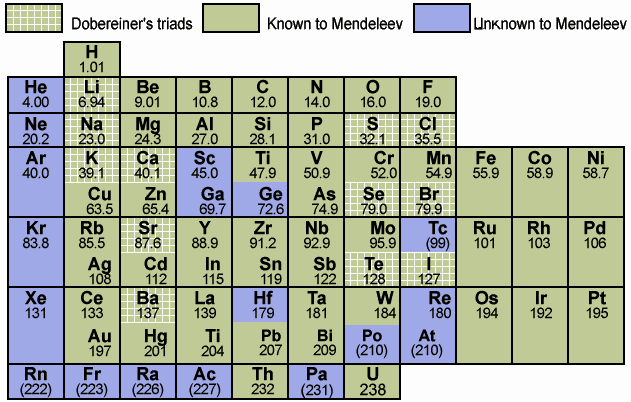
Modern's Periodic Law:In this time which periodic table we use is Modern's periodic table.Modern's periodic law follows that properties of elements are repeated when they are arranged according to their atomic number.This law is successful. Arrangement according to atomic number of elements satisfied their need of to get them in a group where we can get elements of similar properties.There are 18 groups and 7 periods in periodic table. Now each group has atoms of particular properties. First group is consist of alkali metals which are of electropositive nature means these can easily lose electrons .For example electropostive atoms are Lithium ,sodium, potassium etc. 17th group consists of atoms of electronegative nature means the atoms can easily gain electrons. For example electronegative atoms are Fluorine,Chlorine, Bromine etc. Last 18th group is called noble gases .Noble gases are those gases which never react with any element . They are of stable nature.For example Neon,Krypton,Xenon,Radon etc. Lets see modern periodic table.
Like it on Facebook, Tweet it or share this article on other bookmarking websites.

